A guest author blog by Dr Corlett Wolfe Wood
Nearly all organisms rely on other species to perform crucial physiological functions, from digesting food to fighting disease. Beneficial microbes in our own gut, for example, are an essential component of digestive health. Such cooperative interactions between species, known as mutualisms, are evolutionarily ancient and are keystone components of many ecosystems.
But what if it’s difficult to tell friends and enemies apart?
Distinguishing mutualists from harmful parasites is often easier said than done. Many species are attacked by parasites and pathogens that are remarkably similar to their mutualists. In fact, circumstantial evidence raises the intriguing possibility that parasite infection is an unavoidable cost of forming mutualisms. Some species have co-opted genes used to defend against parasites to regulate interactions with their mutualists. Others suppress immune function when attracting mutualists, leaving themselves vulnerable to infection.
Parasites’ resemblance of mutualists poses a major paradox: how do organisms attract beneficial partners while repelling parasites? We hypothesized that the shared genetic control of mutualism and parasitism is likely to create a tradeoff between attracting mutualists and repelling parasites, a phenomenon known as genetic conflict.
The conflict beneath your feet
Although there are compelling reasons to expect that attracting mutualists trades off with resisting parasites, direct evidence is surprisingly scarce. One reason is that mutualists and parasites often live deep inside the host’s tissues (in the digestive system, for example), making them difficult to observe. To get around this problem, we studied microbes that live on plant roots in specialized structures that are visible to the naked eye.
Plant roots are a lot like animal guts: both are responsible for nutrient uptake, and both rely heavily on microbes for help. Legumes—plants like peas and beans, as well as clovers and alfalfa—take the remarkable step of building specialized organs to house their root-associated microbes. If you dig up a bean plant in your garden or a clover in your yard, you’ll find that the roots are covered with small lumps known as nodules (Figure 1, top). Inside these nodules are colonies of mutualistic nitrogen-fixing bacteria. These bacteria provide their legume host with nitrogen—a crucial nutrient for plant growth—in exchange for shelter and carbohydrates.
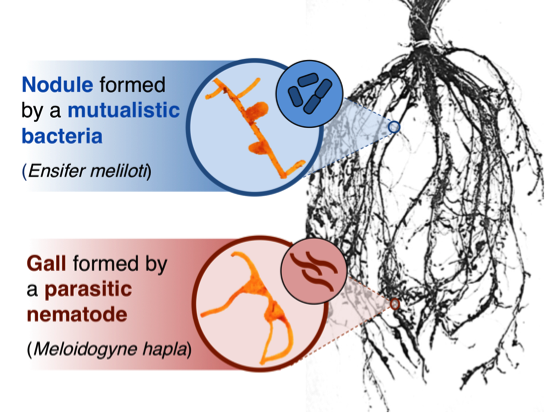
Unfortunately for legumes, mutualistic bacteria aren’t the only microbes that colonize roots. For nearly a century, farmers, botanists, and gardeners have recognized that some nodule-like structures are actually galls formed by parasitic worms called nematodes (Figure 1, bottom). Unlike mutualistic bacteria, parasitic nematodes harm their host. They steal nutrients from the plant without providing any benefit in return.
Nematode galls look so much like bacterial nodules that the Missouri Botanical Garden warns home gardeners to take care not to confuse the two structures. Moreover, past research showed that some of the same plant genes are involved in building both structures. Given the striking similarities between nodules and galls, we wondered whether legumes could form one without forming the other. Can legumes build nodules for mutualistic bacteria without becoming infected by parasitic nematodes?
Clovers that aren’t so lucky
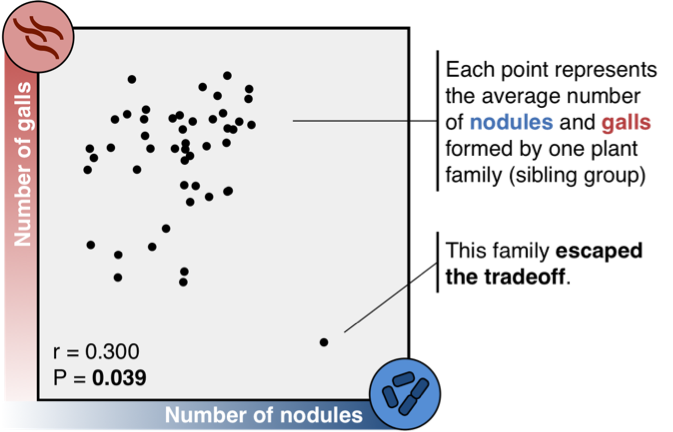
We tested our hypothesis using a small weedy plant called a medick (Medicago truncatula), a close relative of clovers and alfalfa. We grew hundreds of these plants in the rooftop greenhouses at the University of Toronto, and inoculated them with mutualistic bacteria and parasitic nematodes. Just as we predicted, medicks that were good mutualists were more susceptible to nematode infection (Figure 2). Plants that formed many bacterial nodules also formed huge numbers of nematode galls, while those with few nodules formed only a handful of galls. Siblings formed similar numbers of nodules and galls, suggesting the two structures share the same genetic basis.
To our surprise, one medick family escaped the tradeoff between attracting bacteria and repelling nematodes. These plants formed hundreds of nodules but hardly any galls at all (Figure 2, bottom right corner). This family is remarkable because it suggests that although genetic conflict between mutualism and parasitism may be common, it is not inescapable: evolution can find a way out.
What’s next?
My favorite experiments inspire nearly as many new questions as they answer. Next, we want to understand how and why one medick family escaped the tradeoff between mutualism and parasitism. We can start to understand how it escaped by searching for genetic differences between it and the other medick families in our experiment, to find the genes that help it build nodules without also forming galls. We can also learn why it might have escaped the tradeoff by identifying features that distinguish its home environment from that of other medicks. For example, our escapee may come from an environment where parasitic nematodes are hyperabundant. In such an environment, any medick with genes that allow it to form bacterial nodules without also forming nematode galls is likely to outcompete its neighbors.
We suspect that the conflict between attracting mutualists and repelling parasites we discovered in medicks may be a common—but almost entirely overlooked—feature of species interactions. If it is, it complicates how we think about the forces shaping resistance to parasites and cooperation between mutualists. A tradeoff between mutualism and parasitism could constrain the evolution of parasite resistance by reducing the competitive advantage of parasite-resistant individuals. Widespread tradeoffs between mutualism and parasitism may also explain why there so much variation in mutualist quality, when natural selection should otherwise weed out uncooperative partners.
And on a practical level, understanding when and why parasite infection is a collateral cost of mutualism is an important step towards safeguarding the mutualisms we rely on, from the bacteria that nourish plants in our gardens to the microbes that live within our guts.
Dr Corlett Wolfe Wood is an EBB Postdoctoral Fellow at the University of Toronto. Her paper is freely available to read and download in full from Evolution Letters here.