A new paper published in Evolution Letters has shown how the evolution of gene expression differences in males and females can lead to conflict resolution between the sexes. We spoke to lead author Dr Alison Wright to find out more about her latest findings.
EL: The idea of the “battle of the sexes” is familiar to most people, but can you explain why this conflict exists in the first place?
Males and females in many animals have distinct reproductive roles, interests and biology. Evolution sometimes works very differently between males and females, and can push the sexes in opposite directions. Often, this can lead to substantially different phenotypic optima between the sexes, a situation referred to as sexual conflict.
Males and females share an almost identical set of genes and many traits are thought to be encoded by the same genes between the sexes. These shared genetic pathways can pose significant problems when evolution acts differently on males and females, as very often the same gene has different optimal functions in either sex. This is called intra-locus sexual conflict and is thought to be widespread across the entire genome. In theory, unresolved sexual conflict in populations could hinder the evolution of sex differences and therefore incur negative fitness consequences. However, the underlying causes of sexual conflict, and the potential for it to be resolved, has been heavily debated.
EL: What first led you to question whether the evolution of gene expression differences between the sexes could alleviate some of this conflict?
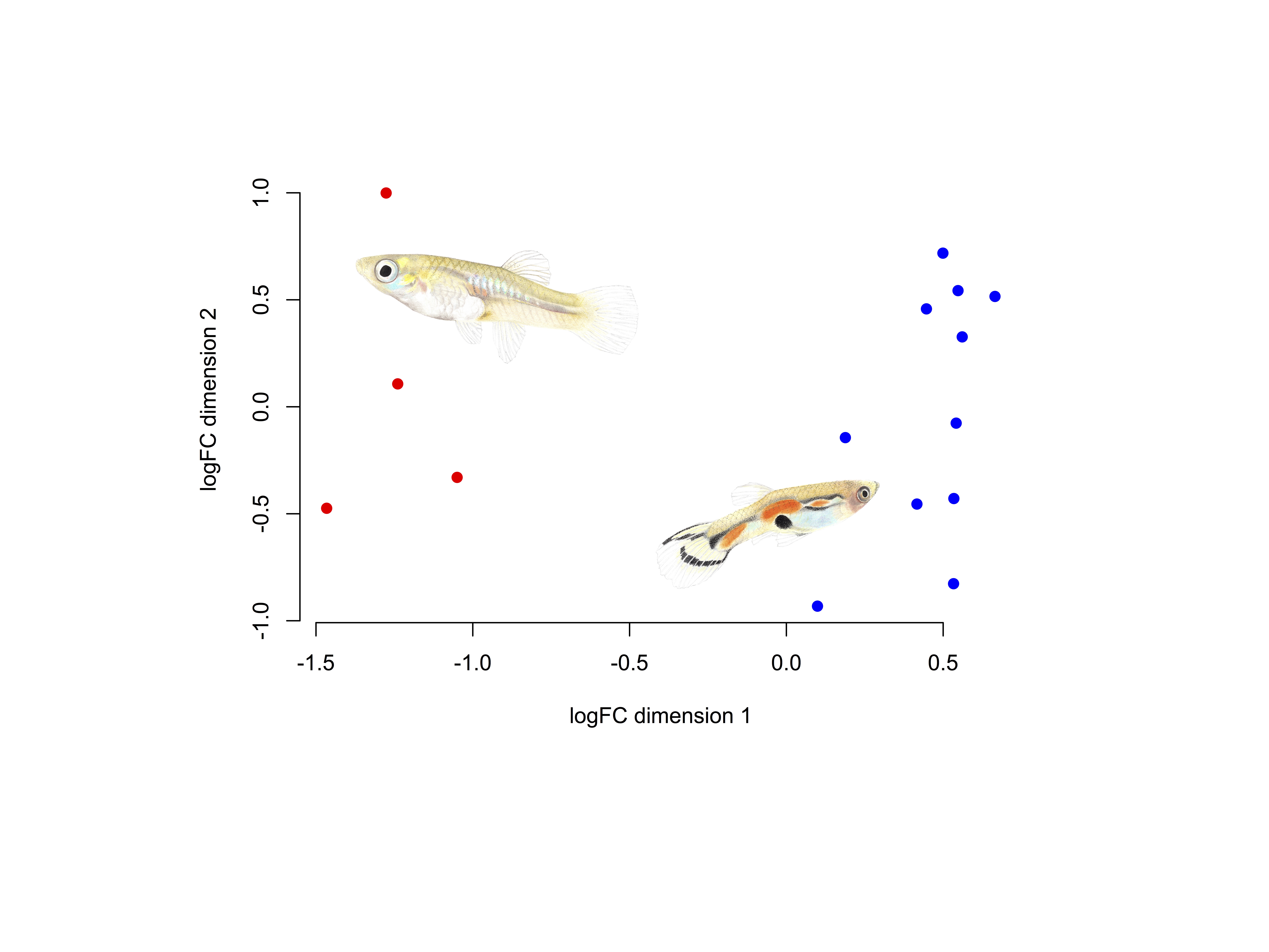
Much of my work to date has focused on the evolution of the sex chromosomes and the role that they play in generating sex differences. Sex chromosomes are the only region of the genome that is shared unequally between the sexes and they are therefore predicted to harbour genes important for sexual dimorphism. For example, in mammals with XY chromosomes, the Y is key to male reproduction and fertility. It is widely assumed that the formation of new sex chromosomes can resolve sexual conflict and recently we set out to test this theory using wild populations of guppies. We found that sexual conflict over colour promotes the formation and divergence of sex chromosomes, consistent with this longstanding theory.
However, sex chromosomes contain only a few genes in many animals, as is the case in the guppy, or are absent entirely, as in many reptiles and fish where sex is determined by the environment. Therefore, sexual conflict for autosomal genes is assumed to occur through the evolution of gene expression differences between females and males, ultimately leading to phenotypic dimorphism. Transcriptional differences can be quite pronounced between the sexes and our previous research has shown that rapid turnover of sex biased expression across a clade of birds is driven by sexual selection as a result of expression changes in males. Recently, there has been considerable debate about the potential role of regulatory evolution to resolve sexual conflict.
We originally developed a set of criteria to identify groups of genes subject to sexual conflict with the aim to test theoretical predictions about their distribution on the sex chromosomes. However, we quickly realised that this was a powerful approach for shedding light on the debate surrounding regulatory evolution and test fundamental predictions about the nature and resolution of sexual conflict across the entire genome!
EL: You carried out your research on a fish species, the Trinidadian guppy. How is sexual conflict manifested in these fish, and what makes them particularly well suited for testing your hypotheses?
Guppies are a classic model species for the study of sexual selection, particularly regarding colour and mate choice. Males are extremely colourful and females prefer to mate with males with large areas of orange carotenoid pigmentation on their tail. However, whilst ornamented males have higher reproductive success, they also attract more predators in the wild. In contrast, males do not prefer to mate with colourful females and therefore colouration genes only increase predation pressure when expressed in females. Additionally, male colour and female preference for colourful males varies with predation regime in the wild.

These landmark studies of sexual conflict over colour in guppies, and the wealth of phenotypic data, make guppies a fantastic model in which to apply genomic tools to study the manifestation and resolution of sexual conflict across the genome.
Sexual conflict can result from different types of selection pressures, including reproductive fitness and survival. The guppy tail combines genes involved in male colouration, which influence reproduction, as well as skin directly interfacing with the environment, which influence predation and survival. Studying the guppy tail therefore offers an exciting opportunity to distinguish the different scenarios giving rise to sexual conflict. Furthermore, that fact that guppies can be kept in the laboratory makes it possible to control many demographic processes which could confound our genomic measures of sexual conflict in the wild.
EL: In your paper, you say that sexual conflict leaves a “signature” in the genetic make-up of a population. Can you explain what that means?
Yes, sexual conflict leaves distinct signatures in sequence data that we can use to understand the nature of sexual conflict and how it is resolved. Intra-locus conflict leads to different versions or ‘alleles’ of the same gene being favoured in males versus females, or vice versa. This can lead to balancing selection, where multiple versions of the same gene are maintained within the population. In turn, this results in elevated genetic diversity which can be measured using Tajima’s D. Tajima’s D is a population genetic test statistic to contrast patterns of genetic diversity to those expected under neutral expectation.
However, we can’t use patterns of elevated genetic diversity to distinguish between sexual conflict arising over reproduction or conflict over survival. To do this, we studied genetic differences between males and females using the population genetic measure inter-sexual FST. Essentially, at the point of conception, we expect different versions of the same gene to be identical between the sexes. However, if an allele affects survival or predation differently between males and females, then this will be reflected in genetic divergence between the sexes in adulthood.
We essentially combined these two signatures of sexual conflict to understand why sexual conflict arises and how it can be resolved.
EL: What were the key findings of your study, and what do you personally think is the most exciting discovery?
We found that the majority of sexual conflict is produced through conflicting selection over reproductive interests of males and females, and that sexual conflict has the potential to maintain genetic diversity through balancing selection. This could explain why patterns of genetic variation are much greater in many animals than might be expected.
We also showed that regulatory evolution, the evolution of gene expression differences between males and females, is a rapid and efficient route to resolving sexual conflict. This result is very exciting as we find that sex-biased expression decouples male and female phenotypes, leading to the evolution of separate sex-specific architectures where alleles have phenotypic effects in one sex but not the other.
EL: What is the importance of these findings for our understanding of how the genetic basis of different characteristics can vary and evolve?
Genetic correlations between males and females arising from sharing a genome are widely assumed to hinder the evolution of sexual dimorphism and adaptation more generally. Our results suggest that the constraints imposed by sharing a genome might not be so difficult to overcome and that the evolution of new genetic architectures are more labile than previously thought. It is also becoming increasingly apparent that sexual conflict can shape broad patterns of genetic diversity across the entire genome. Genetic variation is the source of evolutionary change, and therefore sexual conflict could promote rapid adaptation and evolvability.
EL: What do you think are the most interesting unanswered questions in this field going forward?
This study raises a number of really exciting questions I am keen to pursue further. I am particularly interested in studying sexual conflict in a comparative framework to understand the turnover of sexual conflict and speed at which it can be resolved. How quickly can male and female phenotypes be decoupled? Does this depend on the nature of sexual conflict and conflicting selection pressures? Does sex-linkage promote faster conflict resolution? How does this impact the strength of balancing selection?
Dr Alison Wright is a NERC Independent Research Fellow at the Department of Animal & Plant Sciences, University of Sheffield. Find out more about her research here.


