Yes, according to a new study in Evolution Letters, though the results of its meta-analyses find substantial variation in the degree to which genetic diversity reduces parasitism in host populations. Dr. Amanda Kyle Gibson explains.
Whether we be clinicians, agricultural scientists, epidemiologists, or evolutionary biologists, a shared question among researchers of infectious diseases is: why is an infectious disease so prevalent or so severe in this population of hosts and not this other one? And what can we do about it? One of the most general and long-standing hypotheses to address this question proposes that host populations with reduced genetic diversity experience increased burdens of parasites relative to genetically diverse hosts populations.
You might be familiar with this idea from the notorious epidemics that have decimated crops planted in monocultures, where one or many fields are planted with a single cultivar, or genotype, of the crop plant. These epidemics include the outbreak of coffee rust that decimated Sri Lanka’s coffee industry in the mid-1800’s, the rapid spread of Phytophthora infestans that led to the Irish Potato Famine in the 1840’s, and the recurring threat of fusarium wilt to global banana cultivation. The common explanation for these catastrophes is the genetic homogeneity of crop fields: once parasites can infect one host in a monoculture, they can readily leap to individuals closely related to that host, ultimately infecting the entire field, maybe even all the fields in a region. This argument proposes a solution: if parasites transmit less readily to hosts that are genetically distinct from the original host, then the spread of disease may be slowed in polycultures, where fields are planted with multiple genotypes of the crop plant.
These ideas don’t just matter for agriculture. Endangered species lose genetic diversity as their populations become small and fragmented. This loss of genetic diversity is a prominent hypothesis for the epidemics threatening endangered species like the Tasmanian Devil and the black-footed ferret. The hypothesized protective effect of genetic diversity has even helped us understand fundamental puzzles in evolutionary biology – the prevalence of costly strategies, like sexual reproduction and mating with multiple males, that can maintain or increase genetic diversity in lineages.
To say that I’ve relied on these ideas in my research career would be an understatement. Genetic diversity’s general role as a limit on disease spread is fundamental to major evolutionary ideas, like the Red Queen hypothesis. So whenever I’ve referenced the idea – citing thoughtful perspectives (Haldane 1949; King and Lively 2012; Mundt 2002; Sherman et al. 1988) and compelling experimental tests (Ebert et al. 2007; Meagher 1999; Schmid 1994; Zhu et al. 2000) – I’ve always asked myself, how general is the protective effect of genetic diversity really? How strong is it on average? Does the effect vary? How much? Under what conditions? The answers to these questions matter – they help evolutionary biologists like me decide how much confidence to have in the assumptions we make and give practitioners in applied fields data to determine if the potential benefits of genetic diversification are sufficiently promising to justify the investment. We can arrive at answers to these questions via an approach known as meta-analysis, the synthesis of results from multiple studies to quantify the mean and variance of a variable’s effect.
My coauthor Anna Nguyen and I set off to find the right literature for this meta-analysis. In combing through the literature, we were immediately struck by the incredible variety of studies addressing the relationship between genetic diversity and disease. We slowly developed our literature search protocol, narrowing our focus to studies that quantified some measure of parasitism (e.g. prevalence) in host populations, artificial or natural, that varied in their genetic diversity. We focused on genetic variation between individuals within a population, rather than the genomic heterozygosity of individuals themselves. That factor is for another team to tackle! We ended up with 102 eligible studies, representing investigations of both experimental and natural populations of plant (some crops), vertebrate, invertebrate, and bacterial hosts parasitized by viral, protozoal, fungal, bacterial and animal parasites.
We first focused on experimental studies. In these, researchers would assemble artificial host populations with high diversity by, for example, combining multiple host genotypes or collecting offspring from a female mated with multiple males. Host populations with low diversity instead would have individuals of a single genotype or offspring from a female mated with a single male. Researchers would then expose these populations to parasites, artificially in the lab or naturally in the field. If genetic diversity does protect against parasitism, you’d predict less parasitism on average in the high diversity host populations than the low diversity ones. Our synthesis found strong support for this prediction in the literature. Increasing genetic diversity reduced parasitism by ~20% on average across 25 studies of non-crop hosts. This estimate resembles that from Ekroth et al. (2019), a meta-analysis of a similar set of studies. A reduction in parasitism of 20% is noteworthy, but it pales in comparison to the effect of genetic diversity in crop experiments: across 55 studies, polycultures had ~50% less parasitism on average than monocultures.
Given this effect in experimental populations, we moved on to testing the same idea in observational studies of natural populations. We defined observational studies as those in which the researchers played no role in manipulating the genetic diversity or parasite exposure of the host populations. For example, Meagher (1999) measured the prevalence of nematodes in nine wild populations of deer mice in Northern Michigan, USA. If genetic diversity protects against parasitism, it seems intuitive to predict a negative correlation between population-level estimates of diversity and parasitism. Our synthesis of 22 observational studies did not support this prediction. There are some interesting reasons that we actually expected this result, and I’ll leave those details for the paper. The key points are these: first, a protective effect of genetic diversity may result in strongly positive or negative correlations between population-level estimates of diversity and parasitism, depending on the set of populations you’re looking at. Second, we did find a negative correlation between diversity and parasitism in populations of threatened host species. We’d like to get our hands on more data with which to better test this relationship, but it supports the idea that conservation of genetic diversity is an important consideration in management of threatened species.
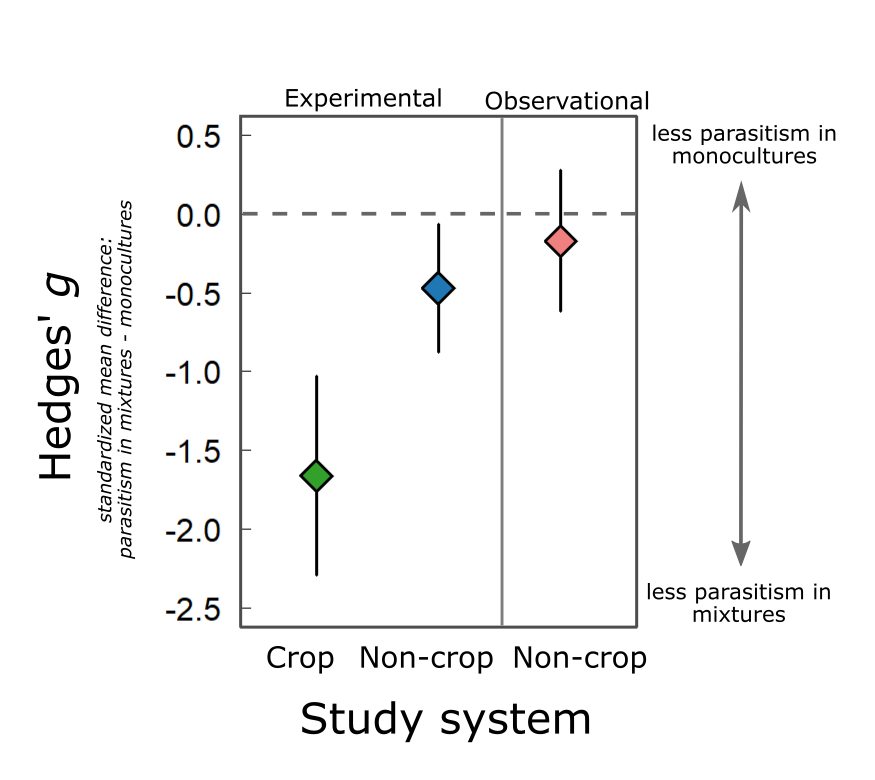
A summary of our results. Hedges’ g is the mean difference in parasitism between high and low diversity host populations, standardized by the variation between replicate populations. Negative values mean that genetically diverse populations have less disease on average. The magnitude of Hedges’ g gives us a sense of the magnitude of the effect. The magnitude of the mean effect for experimental crop studies is consistent with a strong effect of genetic diversity on parasitism, while the magnitude of the mean effect for experimental non-crop studies is moderate. We see no mean effect in observational studies.
The results of this study give substantial weight to the long-standing hypothesis that genetic diversity of host populations can limit parasitism. Our synthesis of agricultural experiments underscores polyculture as a sustainable measure for controlling disease and maintaining yield in crop fields. Our synthesis of experiments in non-agricultural systems supports hypotheses that propose parasites as an explanation for the puzzling prevalence of strategies like sex and polyandry. Our analyses also warn of the danger posed by disease outbreaks when human actions rob natural populations of their genetic diversity. We hope our findings spur the use of genetic diversification as a tool in disease management and motivate research into the curious gaps in the literature revealed by this synthesis.
Dr. Amanda Kyle Gibson is an assistant professor at the University of Virginia. She co-authored the original article with Anna Nguyen, a third-year undergraduate researcher. It is freely available to read and download from Evolution Letters.
References
Ebert, D., F. Altermatt, and S. Lass. 2007. A short term benefit for outcrossing in a Daphnia metapopulation in relation to parasitism. Journal of the Royal Society Interface 4:777–785.
Ekroth, A. K., C. Rafaluk-Mohr, and K. C. King. 2019. Host genetic diversity limits parasite success beyond agricultural systems: a meta-analysis. Proceedings of the Royal Society B 286:20191811.
Haldane, J. B. S. 1949. Disease and evolution. La Ricerca Scientifica 19 68–76.
King, K. C., and C. M. Lively. 2012. Does genetic diversity limit disease spread in natural host populations? Heredity 109:199–203.
Meagher, S. 1999. Genetic diversity and Capillaria hepatica (Nematoda) prevalence in Michigan deer mouse populations. Evolution 53:1318–1324.
Mundt, C. C. 2002. Use of multiline cultivars and cultivar mixtures for disease management. Annual Review of Phytopathology 40:381–410.
Schmid, B. 1994. Effects of genetic diversity in experimental stands of Solidago altissima–evidence for the potential role of pathogens as selective agents in plant populations. Journal of Ecology 82:165–175.
Sherman, P. W., T. D. Seeley, and H. K. Reeve. 1988. Parasites, pathogens, and polyandry in social Hymenoptera. American Naturalist 131:602–610.
Zhu, Y., H. Chen, J. Fan, Y. Wang, Y. Li, J. Chen, J. Fan et al. 2000. Genetic diversity and disease control in rice. Nature 406:718.