A new study published in Evolution Letters has revealed a positive correlation between male and female reproductive success in Japanese quail. Here, lead author Dr Joel Pick describes the unique study system used for this work, and explains what the findings tell us about individual variation in reproductive investment.
Why variation in reproductive function exists has long puzzled evolutionary biologists. To address this question, Dr. Joel Pick and colleagues at the University of Zurich, Switzerland, created replicated, artificial selection lines for female reproductive investment using the Japanese quail (Coturnix japonia), a precocial bird. After only a few generations, the researchers saw a strong divergence in egg size between the lines selected for high and low investment, demonstrating that female reproductive investment is able to respond rapidly to selection. Further work by the team demonstrated the strong positive effect of female egg investment on chick size and survival, and more recently, the buffering effects on this investment on inbreeding depression.

If egg investment has such positive effects on offspring, and has a heritable basis, why then do we see variation in egg size in natural populations? From a traditional life-history perspective, we would assume that egg investment is costly to the mother, and these costs help to maintain this variation. Indeed, using these lines, the team from Zurich were able to show that producing larger eggs is metabolically costly, with females needing to have large reproductive organs and a higher metabolic rate to produce this higher reproductive investment. This investment also resulted in high investment females having a smaller cerebellum, a brain region associated with diverse cognitive functions.
This story has, thus far, ignored males. As males and females share the majority of their genes, any selection on females will likely impact upon males. Indeed, many studies have shown that in traits shared between the two sexes, selection on one sex results in a correlated response in the other sex. As reproductive organs are highly functionally divergent between the two sexes, selection may act in a different way in the two sexes, leading to conflict. However, theory suggests that strong sexual dimorphism, such as that seen in reproductive organs, indicates that past sexual conflict has been resolved. Under this hypothesis, we would expect that selection on female reproductive investment has little impact on the males.
In a new study published in Evolution Letters, the team from Zurich tested these hypotheses by assessing the reproductive success of males from both lines in two environments. Firstly in a non-competitive environment, when a single male and female were paired up, and secondly in a competitive environment where groups of males and females were kept in aviaries. Surprisingly, the team found that males from the high investment lines had a higher reproductive success (fertilised more eggs) in both environments. To understand this effect more, the size of both testis in these males were measured. Although total testis size, which is commonly thought to reflect the sperm producing ability of males, did not differ between the two lines, testis asymmetry did, with the left testis being relatively larger in males from the high investment line. Furthermore testis asymmetry was correlated with the reproductive success of males in both settings.
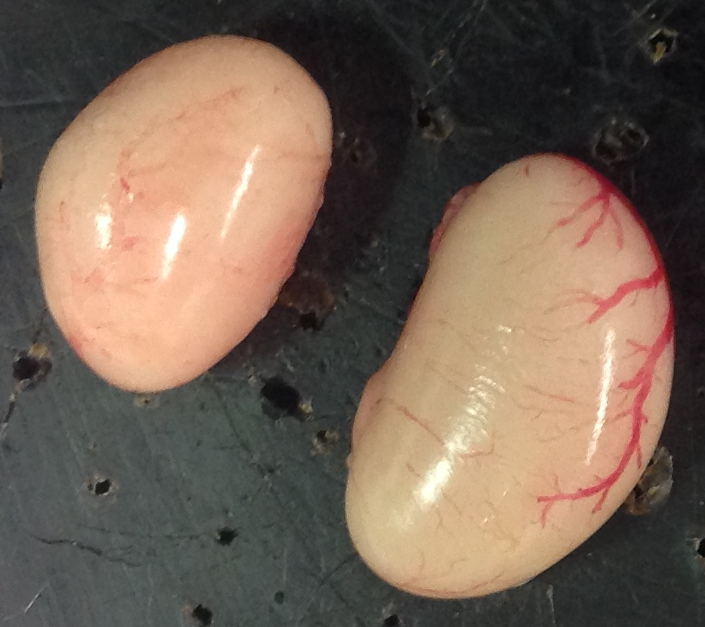
Why the left testis? Well, in most species, female birds have only one set of reproductive organs on their left side (rather than one on each side, as seen in many other taxa). This is thought to have evolved as an adaptation to flight. In many bird species, testis are asymmetric, typically with the left testis being larger. One hypothesis is that the asymmetry seen in males is a result of similar (albeit more extreme) asymmetry in females. The team from Zurich had already shown that, in order to produce larger eggs, females from the high investment line have larger reproductive organs on their left side. Together, this indicates that selection acted on a joint developmental basis of male and female gonads. This ties in with recent developmental work showing that, even after sexual differentiation, both sexes show a difference in gene expression and stem cell number between right and left gonads. It further indicates that there might be functional differences between the two testis, linking to other recent work that has shown that it may not be the size of the testes that matters, but what they are made of.
So if the reproductive success of males and females is positively correlated, how does this help explain the maintenance of variation in reproductive function? Surely this means that there is an even larger selective pressure acting on these traits? Well, the presence of this positive correlation between the two sexes also means that anything acting to constrain the evolution of reproductive function in one sex, will also constrain its evolution in the other sex. Several studies have shown that, for example, male reproductive success and longevity are traded-off against each other, meaning that forces acting in males may hinder the evolution of female reproductive investment. From the other perspective, given the costs of reproductive investment that have been shown in these selection lines, constraints to female reproductive investment may also be hindering the evolution of male reproductive success.