A new study published in Evolution Letters examines how mating systems have been shaped by historic biogeographic events. Lead author Matt Koski explains the findings and what they tell us about evolutionary responses to global climate change.
Historic climate oscillations have been a driving force in shaping the geographic distribution of species. During the last ice age, many plants and animals were restricted to suitable habitat called glacial refugia. In the Northern Hemisphere these refugia often existed at southerly latitudes. As the global climate warmed and glaciers receded poleward, species expanded out of these refugia into newly suitable habitat. Picking up and moving to a new location should have important impacts on the genetic diversity of populations, their evolution, and maybe even their fate. Do we find a legacy of historic range expansion in contemporary populations and does this provide insight into potential responses to contemporary climate change?
Colonization of new habitats often involves just a few individuals, resulting in reduced genetic diversity relative to source populations. Repeated founder events during range expansion should therefore reduce diversity in populations farthest from glacial refugia. Another genetic consequence of range expansion can be the accumulation and fixation of harmful mutations in populations at the expanding range edge. This is called the ‘expansion load’ and it can reduce the fitness of populations near expanding edges. Geographic range expansion should also have cascading effects on a fundamental aspect of reproduction in hermaphroditic organisms — whether to find a mate (outcross) or mate with themselves (self-fertilize). In small founding populations, individuals that are able to self-fertilize may be favored because they don’t need to find a mate to reproduce. Additionally, in populations with low genetic diversity (like those far from glacial refugia) rare harmful mutations that reduce the performance of inbred offspring may be lost through founder events. These ideas both suggest that self-fertilization evolves more easily in populations with low genetic diversity.
To test for these consequences of range expansion, we studied populations across the geographic range of American bellflower (Campanula americana)—a charismatic flowering plant from eastern North America (Fig. 1).

An important first step in this work was to figure out where the species likely hid out during periods of glaciation. We had several ideas given past work in this species, but we wanted to fully leverage our genomic data. We ended up borrowing a technique taken from another discipline (the “time difference of arrival” approach, which can be used to solve some navigation problems). Using this method, we analyzed the geographic distribution of derived alleles to establish that the most likely origin of range expansion (i.e., the glacial refugium) was in the Southern Appalachians in present-day southeastern Kentucky (Fig. 2A). Populations farther from this point were effectively smaller (Fig. 2B), reflecting a history of bottlenecks. Such a finding is expected whenever the expanding range edge is pushed forward by the movement of few individuals again and again through time.

After we established the location of a glacial refugium, we then interpreted genetic differences among populations in this light. We found that populations at the expanding range edge also displayed reduced fitness when crossed with members of their own population relative to crossing with other populations. This was the phenotypic expression of mutation accumulation and reduced genetic diversity at the expanding edge. Thus, the expansion load depressed average population fitness.
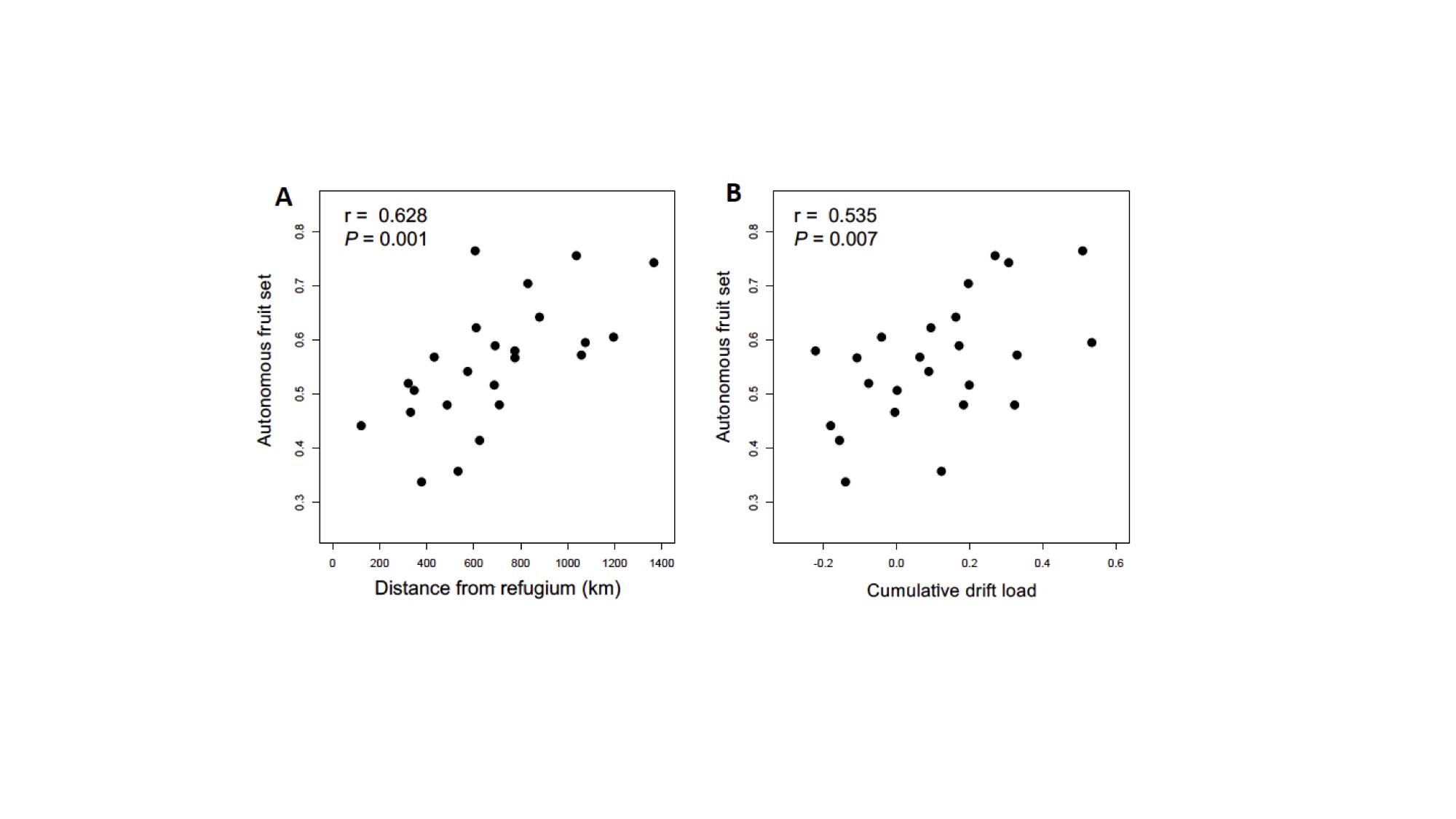
We then turned our attention to geographic variation in the ability to self-fertilize among populations, or their ability to produce fruit in the absence of pollinators. Autonomous fruit set was very tightly linked with both the distance from the glacial refugium and genetic drift load (Fig. 3). That is, populations that colonized farther from the glacial refugium have an elevated ability to self-fertilize. This result strongly implies that range expansion has structured geographic variation of the mating system. Previous work found that pollinator activity and the number of plants in a population (i.e., mate availability) were unrelated to autonomous selfing ability in American bellflower. Thus, historical processes shaped variation in the potential for selfing, but this may not necessarily be adaptive in contemporary populations.
In the end, does this work in bellflower change the way we think about evolution? We think it draws attention to older historical events as determinants of plant sexual systems. Since the middle of the 20th century, a wealth of studies that have shed important light on why plants outcross or self. While the ecology of contemporary populations (e.g., mate availability, or pollinator availability) is clearly important, our bellflower study shows that historic colonization can explain much variation in the geography of plant reproduction. In an even broader perspective, this work highlights some of the potential costs of range expansion that are likely to haunt species that move into new habitats made suitable by warming climates. Mutation accumulation has the potential to restrict species’ ranges and limit adaptation to contemporary climate change. Our work only hints at these possibilities. Future work in evolutionary biology will of course see whether these ideas stand the test of time.
Matt Koski is Assistant Professor at the Department of Biological Sciences, Clemson University. The original study is freely available to read and download from Evolution Letters.