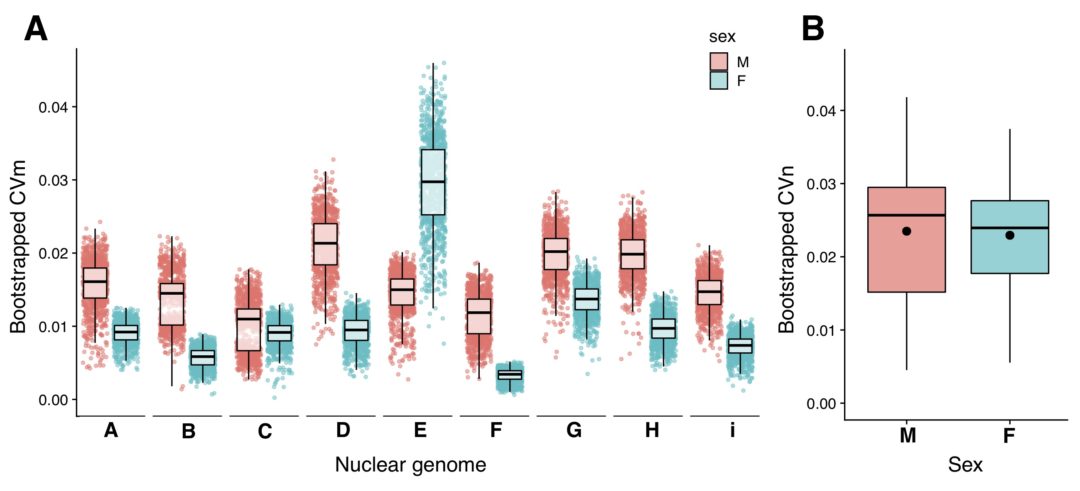
A new study in Evolution Letters provides experimental evidence that predation drives changes in threespine stickleback armor traits. Here, author Dr Diana Rennison describes her experiment and its interesting results.
For quite some time it has been known that differences in ecological factors between habitats drive evolutionary divergence and can lead to the generation of new species. However, most of the time we do not know the sources of natural selection that drive the evolution of specific adaptive traits. It is also often the case that trait changes are driven by multiple ecological factors and interactions between them, which makes it difficult to determine the individual contributions of each factor. As a result, it remains unclear how species interactions, such as predation or resource competition, contribute to species diversity. To investigate whether differential predation could be an important factor that drives diversification of traits and their underlying genes we experimentally manipulated the presence of a native predator of threespine stickleback.
Threespine stickleback (Gasterosteus aculeatus) are a small fish distributed throughout most coastal regions of the northern hemisphere. Stickleback can be found in marine habitats, where they have existed for millions of years. They can also be found in freshwater habitats, which they colonized 10 – 12,000 years ago after the last ice age. Within freshwater, stickleback have adapted to thousands of lake and stream habitats. Interestingly, within five lakes in British Columbia Canada two distinct species of threespine stickleback have evolved and coexist in adjacent habitats. In these lakes there is a benthic species that is larger, deep bodied, and feeds in the near shore environment on small insect larvae, snails and clams. There is also a smaller and more streamlined limnetic species, that inhabits the open water, where it feeds on zooplankton. Previous work has shown that competition for resources between the two species likely drove them to occupy the two different habitats. Once in their new habitats, benthics and limnetics encountered distinct predators. In the near shore, insect predators are more common, whereas in the open water vertebrate predators (birds and cutthroat trout) are more common.
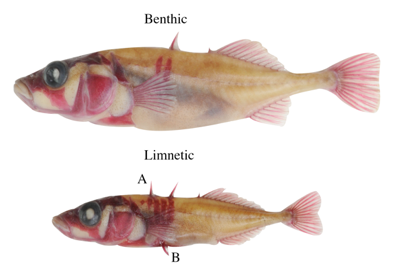
Benthic and limnetic stickleback differ in many behavioural and morphological traits. However, one striking difference is in the amount of bony armor that they possess. Limnetics have two long dorsal spines along their back and two spines protruding from their pelvis, they also have 5–9 bony plates along each side of their flank. In contrast most benthic fish have lost their first dorsal and pelvic spines, and often have only 0 – 4 bony plates. This armor is thought to protect against predation by toothed or gape limited predators, such as predatory fish (e.g. trout) or birds. Thus, the differential predation they experience is hypothesized to have driven the divergence of these armor traits. Previous work in threespine stickleback had identified the genes responsible for variation in the spine traits. A mutation at the Pitx1 locus confers loss of the pelvic spines and girdle and reduced dorsal spine length results from changes in the Msx2a gene.

To test the idea that predation is the source of selection driving changes in the armor of benthic and limnetic stickleback, we conducted a manipulative experiment. In order to isolate and estimate the selection due to cutthroat we conducted the experiment in artificial ponds that did not naturally contain any fish species. We used hybrid benthic-limnetic stickleback as the target of selection and manipulated the presence or absence of cutthroat trout. The reason for using hybrid stickleback was to increase the amount of variation available for selection to act upon, which would make it easier for us to detect an effect. Some hybrid fish had very benthic armor, some had very limnetic armor and some had intermediate trait values.
In the spring of 2012, we introduced the first generation of hybrid fish (F1s) into the ponds. They immediately started to breed and created the second generation of hybrids (F2s), our targets of selection. At this point we took an initial sample of these second-generation fish, to determine the starting frequencies of the armor traits and their underlying genes. We then introduced two adult cutthroat trout into half of the experimental ponds, with the remaining ponds acting as our trout free controls. The following spring the second generation of hybrid fish bred, creating the third generation (F3s). In the fall of 2013, after nearly a year of natural selection we sampled the phenotypes and genotypes of this third generation.


To estimate the evolution that had taken place we compared the phenotypes and genotypes of the fish collected before (F2s) and after (F3s) one year of differential natural selection. By comparing the evolutionary response of the ponds that contained trout to the control ponds we were able to determine whether trout drive the divergence of armor. Remarkably we found that in as little as one year the addition of trout caused a habitat shift of the sticklebacks in the ponds and evolutionary divergence in armor phenotypes and in allele frequencies at the two genes controlling spine length.
This study provides one of the first examples in which the evolution of a phenotypic trait has been linked to both the source of selection and the underlying genes. Our findings also expand the known role of biotic interactions, such as predation, on species diversification and suggest that perturbations to a food web could have cascading effects on the evolution and persistence of species.
Dr Diana Rennison is a currently a postdoctoral fellow in the Peichel Lab at the University of Bern. This fall she will be starting her own lab at the University of California San Diego. The original study is freely available to read and download from Evolution Letters.