New research published in Evolution Letters demonstrates that in fruit flies, female resistance to sexual conflict is dependent on food availability. Lead author of the study, Dr Wayne Rostant, tells us more.
In species which reproduce sexually, i.e. either with separate sexes (or sex functions in the case of hermaphrodites) it is common to find that life-histories and strategies that would optimise female evolutionary success are quite different to those which optimise male evolutionary success. The traits upon which these optimal strategies are based are very often shared by males and females, however, setting up a tension between the sexes. This results in what is known as sexual conflict, defined broadly as sexually antagonistic selection on shared traits. One common example of this is the conflict seen in many species over mating rate, where the optimal rate for males far exceeds that for females. In other words, males can maximise their lifetime reproductive output by mating often whereas females maximise their output by mating one or a few times. Sexual conflict is revealed in the evolutionary to and fro of adaptive response and counter response described as an evolutionary ‘arms race’ between the sexes. An adaptive response to selection which favours males but harms females could result in the evolution of counteradaptations in females to minimize the potentially costly side effects.
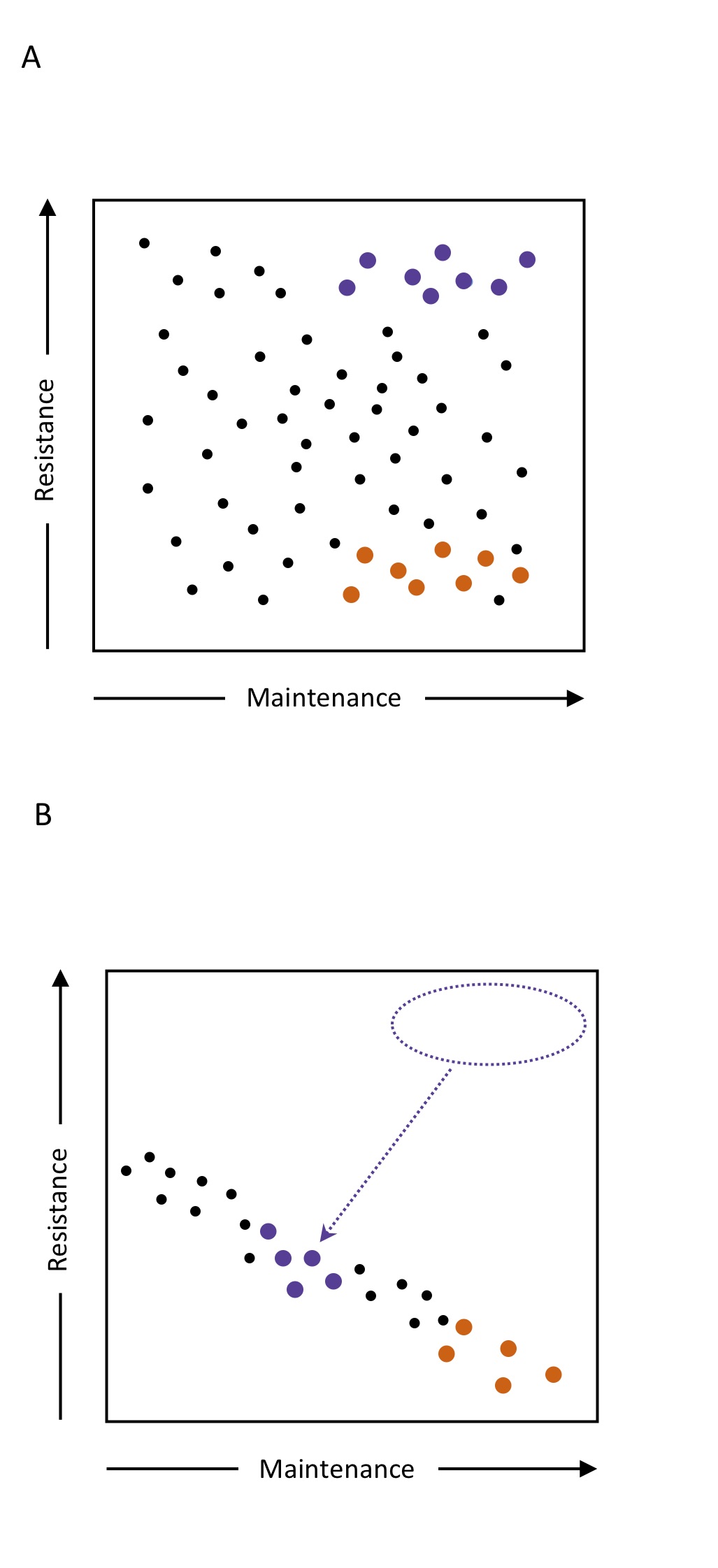
However, this evolutionary ‘arms race’ doesn’t happen within a vacuum. Ecological context is important. Organisms need to survive to reproduce and, given the necessary energetic trade-offs inherent in life-history theory, we should recognize and emphasize the influence of resource levels and condition dependence (i.e. how sensitive traits are to resource levels) when we examine sexual conflict. This is of fundamental importance because resource levels may significantly influence and even reverse the outcomes of sexual conflict. However, to date, we lack empirical tests of this key idea.
This motivated our research on the effects of diet on the evolutionary outcomes of elevated courtship and mating within the fruit fly Drosophila melanogaster. The observed costs of mating in female fruit flies is a relatively well-known example of sexual conflict. Past work by Dr Stuart Wigby and Professor Tracey Chapman (Oxford University) had shown that an evolutionary history of elevated sexual conflict can lead to the evolution of resistance to costs of mating in females. In this scenario, females exposed over time to elevated courtship and mating frequencies evolve the ability to ameliorate these effects such that they can express higher survival when continually exposed to males. In a replay of that experiment we set up various fruit fly populations in which we manipulated the adult sex ratio to either be male-biased, equal-sex ratio or female-biased. These treatments are proxies for high-conflict, medium-conflict and low conflict respectively, as they result in females experiencing correspondingly high, medium and low rates of courtship and mating. The key elaboration this time around involved a simultaneous manipulation of adult resource levels where half of the populations were given a high yeast diet and the other half a low yeast diet. We then allowed these populations to evolve under these manipulations for several generations before conducting life-history tests at different time-points.
Observation of in-situ (that is, in the population cages) behaviour showed no effect of the resource levels on the amount of attention received by females, thus allowing us to test, in well-adapted populations experiencing ostensibly similar selection pressures, the hypothesis that resource levels constrain female responses to elevated sexual conflict. High-conflict females from rich-resource regimes, unconstrained by trade-offs with somatic maintenance, were expected to have the capacity to express resistance responses. In contrast, under poor resource regimes, such females are expected to evolve resistance to sexual conflict only by trading it off against somatic maintenance i.e. the business of simply surviving. Hence, our first prediction was that elevated sexual conflict would restrict the evolution of male harm resistance to resource-rich regimes, because only in these would females have the capacity to respond to selection by investing in somatic maintenance and resistance. The general lack of male survival responses to sexual conflict observed in the previous Wigby and Chapman study and the finding that life history traits in males can show limited responses to proximate diets generated a second prediction: that the outcomes would be sex specific.
We tested these predictions with standardized assays on flies descended from these experimental evolution populations. We determined lifespan, ageing, and fitness for both sexes of each regime under “no conflict” (once mated individuals—to reveal investment in baseline somatic maintenance) and “conflict” (continually mated individuals—to indicate investment in resistance) conditions. Consistent with the Wigby and Chapman study, in resource-rich regimes the high-conflict females evolved resistance to continual exposure to males, being longer-lived than low-conflict females when tested in the “conflict” assay. Under the “no-conflict” conditions there was no difference in baseline survival, consistent with the idea that responses evolving under nutritional abundance experienced no trade-offs with resistance. This contrasted with the results from the poor resource regimes, where the ability of high-conflict females to evolve resistance to males was severely compromised and they also showed lower baseline survival than low-conflict females. This suggested high conflict females traded off somatic maintenance against any limited resistance they had evolved in response to sexual conflict. As predicted, we also found males generally unresponsive to the sex-ratio and resource treatments.
Overall, these findings provide experimental support for the hypothesis that evolutionary responses to sexual conflict are critically dependent upon resource levels. Specifically, we have shown that resource availability can constrain the expression of responses to elevated sexual conflict and in doing so reveal underlying trade-offs. The work highlights the key, general role of resources such as food in determining the expression of responses to selection.
Dr Wayne Rostant is an evolutionary biologist at the University of East Anglia. The original study is freely available to read and download from Evolution Letters.