A new study published in Evolution Letters has shown that testosterone shapes the underlying genetic parameters determining how populations respond to selection. Authors Tyler Wittman and Robert Cox tell us more.
In many species, females and males look strikingly different. This phenomenon of sexual dimorphism is quite common, but it is puzzling when you consider that both sexes share most of the same genes. For example, male brown anole lizards weigh twice as much as females and have a large and brightly colored throat fan (dewlap) that is ten times smaller in females. Yet, the vast majority of an anole’s genes are present in both sexes. This means that natural or sexual selection for larger size or brighter coloration in males should lead to the inheritance of these same traits by females. How do females and males evolve to be so different despite sharing most of the same genes?
We can estimate the extent to which shared genes are likely to constrain evolution by measuring the degree to which traits are genetically correlated between females and males. For example, the hue of the anole dewlap is strongly genetically correlated between the sexes. This means that males with redder dewlaps will have sisters, mothers, and daughters with redder dewlaps, whereas males with yellower dewlaps will have female relatives with yellower dewlaps. However, genetic correlations for highly sexually dimorphic traits, such as the size or brightness of the dewlap, are much weaker between females and males. How are these genetic correlations weakened to facilitate the evolution of sexual dimorphism?
One possibility is that sex hormones such as testosterone, which circulates at higher levels in males than in females, enables the sexes to express their shared genes in different ways. Although we did not measure gene expression in this study, it is well known that testosterone and its receptor can interact with DNA to increase or decrease the expression of hundreds of genes at a time. Therefore, we decided to ask whether a single hormone could be responsible for simultaneously reducing genetic correlations for a variety of sexually dimorphic traits.
Testing this idea required breeding hundreds of lizards for more than a year to produce over a thousand offspring (which would not have been possible without the help of many dedicated UVA students!). Next, we split each anole family so that half of the siblings received a slow-release testosterone implant at three months of age, while their brothers and sisters received an empty implant as a control. In juvenile males, testosterone levels are just beginning to increase at this age (similar to puberty in mammals). What would an increase in testosterone do to juvenile females, who naturally have low testosterone levels throughout their lives?
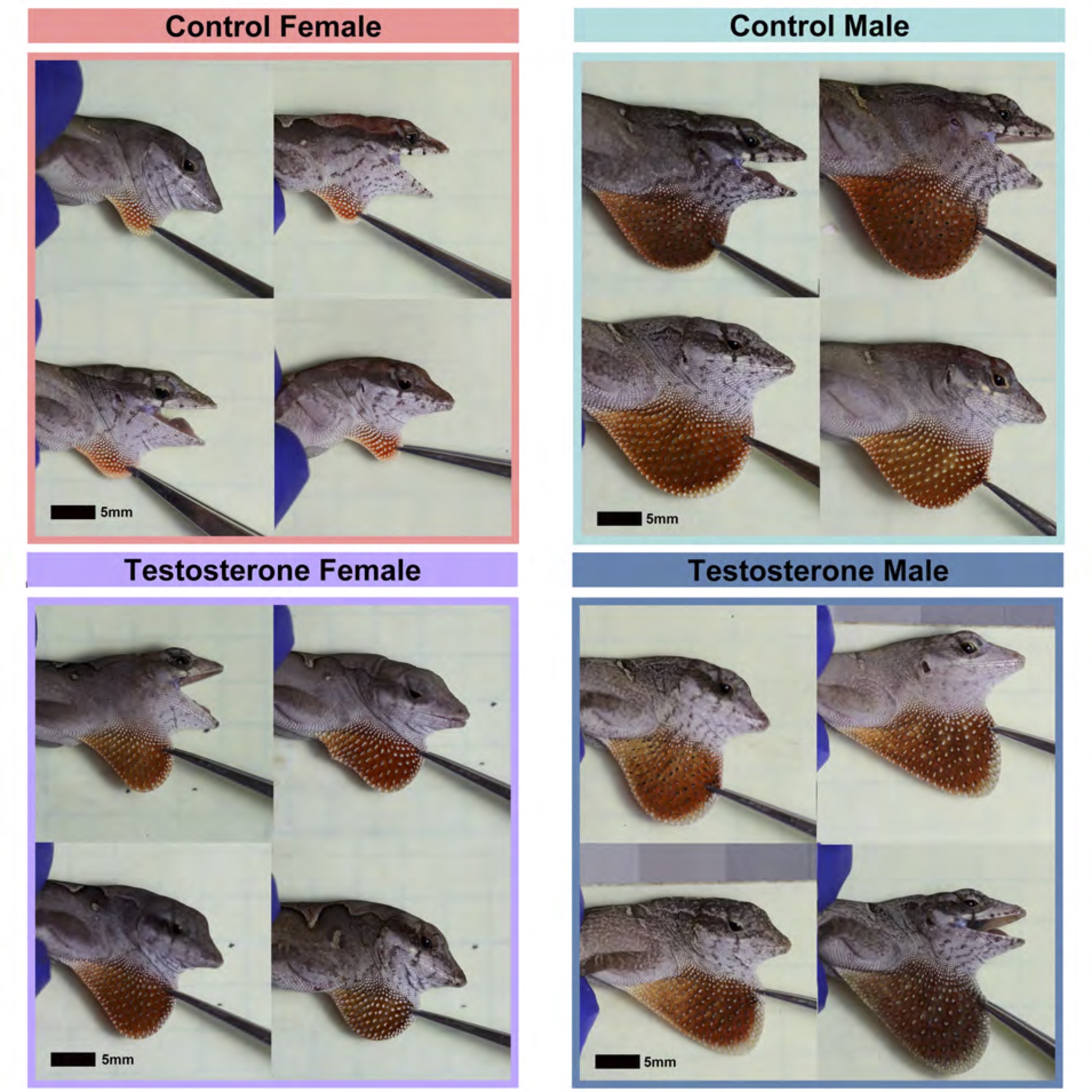
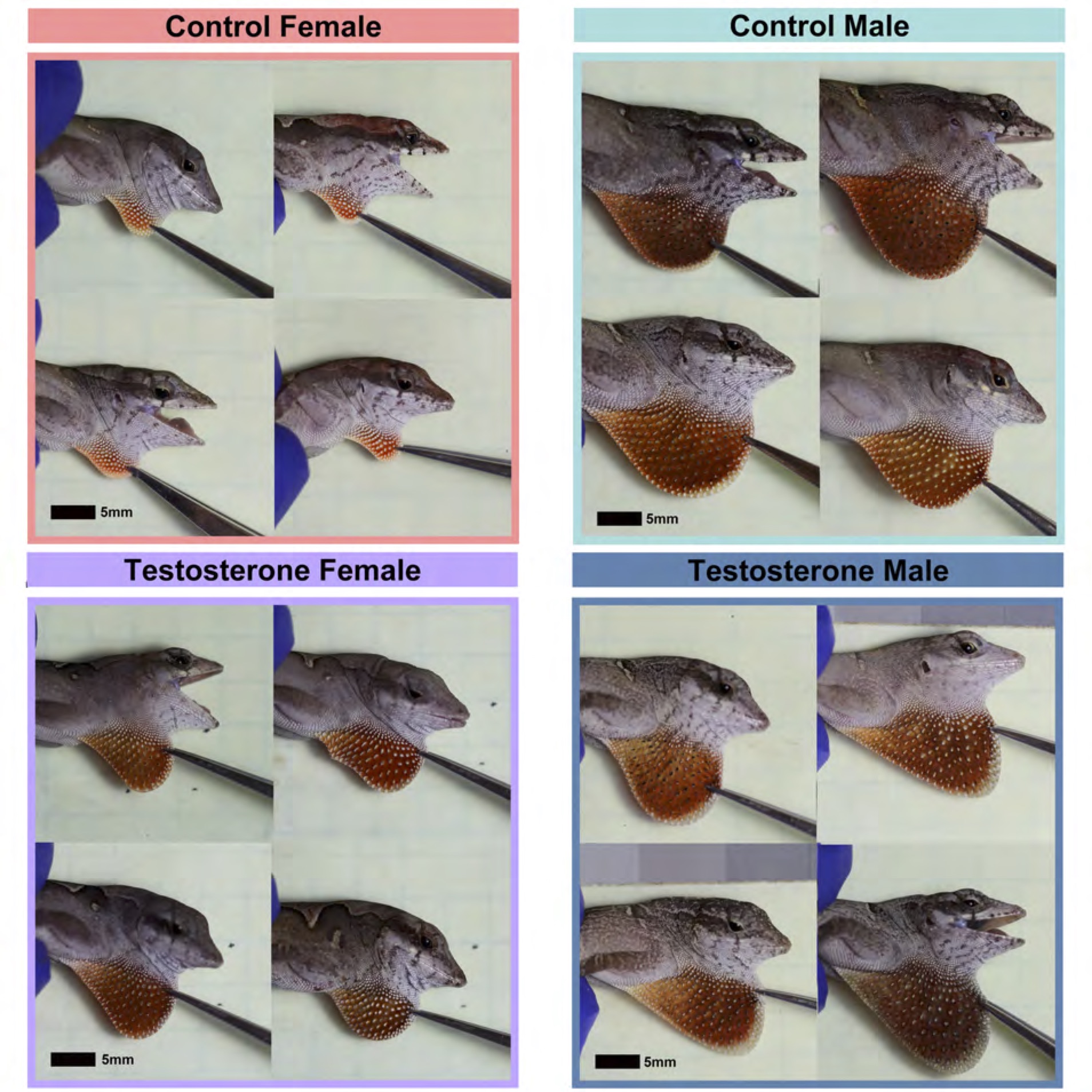
From the image above, you can see the dramatic effects of testosterone on the size and color of the dewlap in females. Testosterone also significantly enhanced the growth of females. In other words, testosterone made females more similar to males in terms of body and dewlap size, and it eliminated most sex differences in dewlap color. These results are rather striking, but not exactly newsworthy – it is already well known that testosterone regulates sex differences in growth and coloration in a variety of mammals, birds, reptiles, amphibians, and fish.
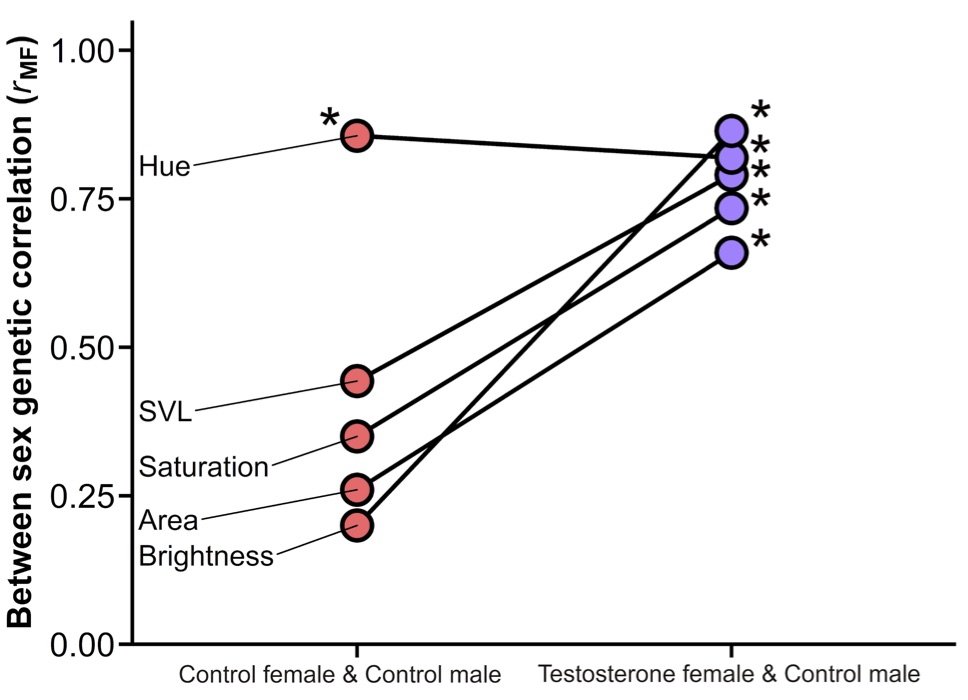
Because we knew the genetic relationships of all the anoles in our study, we were able to go a step further to provide the first direct evidence that testosterone weakens genetic correlations between females and males. As seen in the figure below, genetic correlations between control females and control males are weak for most traits except dewlap hue, suggesting that natural sex differences in testosterone allow the sexes to express their shared genes in different ways. However, genetic correlations are significantly higher between females treated with testosterone and these same control males, as expected if the sexes share the same genes and the resulting genetic correlations are only weakened when they differ in testosterone levels.
Going a step further, we simulated sex differences in natural selection to show that the weak genetic correlations between control females and control males are much less likely to constrain their evolution than the strong correlations that arise when both sexes have high testosterone levels. We also found that control females differ from control males in the way that pairs of traits are genetically correlated with each other. However, when females receive testosterone, their traits become genetically correlated in a way that resembles males.
Our study is the first to directly show that hormonal pleiotropy – when one hormone influences multiple traits – structures patterns of genetic correlation between the sexes and between different traits. Because these patterns of genetic correlation should influence how females and males evolve in response to natural and sexual selection, our study suggests that hormones like testosterone play important roles in evolution, helping the sexes evolve dramatic differences despite sharing a genome.
Tyler Wittman is a PhD candidate in the lab of Dr Robert Cox at the Department of Biology, University of Virginia. The original article is freely available to read and download from Evolution Letters.