A new study published in Evolution Letters provides support for the ‘Mother’s Curse’ hypothesis in a more generalisable way than previously demonstrated. Lorcan Carnegie, who is an author on the paper, gives us an overview of the findings.
Mitochondria are organelles that drive the production of cellular energy and metabolic substances in many forms of life. Crucially, mitochondrial function is dependent on interactions of both the nuclear and mitochondrial genomes.
That’s all well and good, but what is the Mother’s Curse? Well, here’s a quick break-down for those of you who are unacquainted with this topic: mitochondria are not passed on to the next generation by both parents – they exhibit strict maternal inheritance across most species. The ‘Mother’s Curse’ hypothesis suggests that, as all adaptations that are beneficial for females are not also good for males, this evolutionary bias (or ‘sex-specific selective sieve’) enables the accumulation of male-harmful mutations in mitochondrial DNA (Fig. 1). Okay, so why don’t males go extinct?… Well, it is believed that nuclear alleles arise that compensate for male-harming mitochondrial DNA mutations. However, given the difference in evolution rates between mitochondrial and nuclear genes (the nuclear genome mutating at a much slower rate) this grand rescuing force is thought to often lag behind the effects of the Curse.
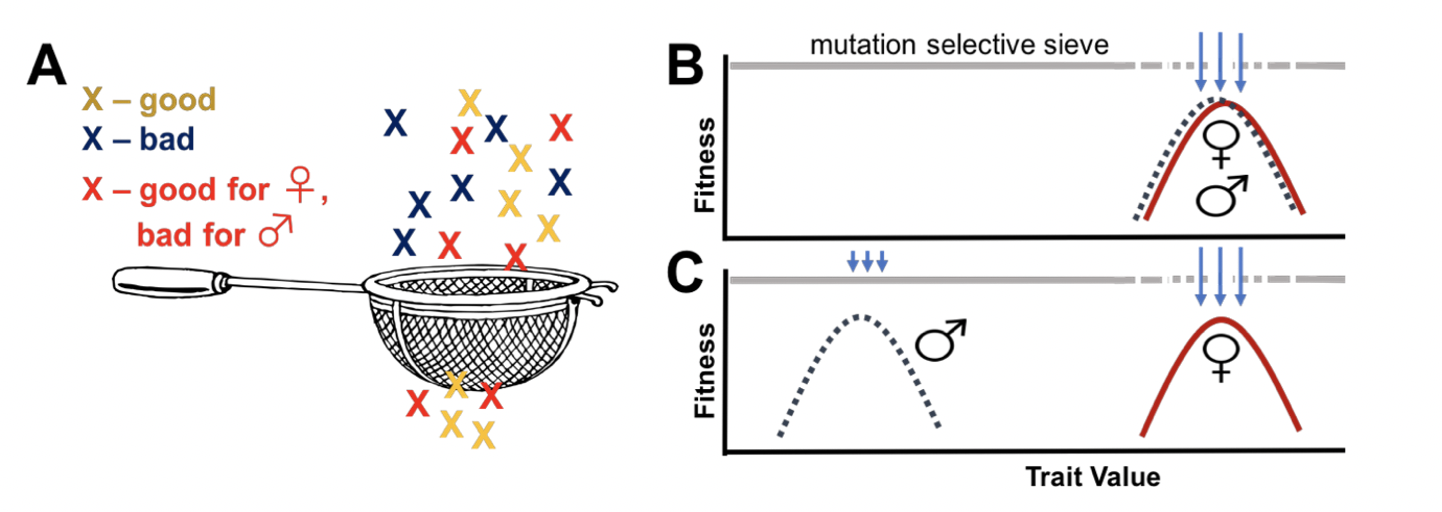
There are two testable predictions associated with the presence of the Curse. A first prediction is that traits with high metabolic demands (i.e., rely a lot on mitochondrial function) whose expression varies between the sexes (i.e., different selection on female mitochondrial DNA than males) should be most to be affected by the Mother’s Curse. A second prediction is that, because populations should harbour mitochondrial genomes comprising male-harmful mitochondrial mutation loads, we should see greater levels of mitochondrial genetic variance in its males than in its females. While different populations should evolve along their own population-specific trajectories and accumulate their own distinctive pools of male-harming mitochondrial DNA mutations, such mutations can be unmasked by placing the mitochondrial genome alongside a foreign nuclear genome!
So, what proof is there that the Curse truly exists? There has been anecdotal evidence for male-deleterious mitochondrial mutations in humans, mice, and fowl. Additionally, some past experimental work using fruit flies (Drosophila melanogaster) provides some support for the hypothesis. However, these past Drosophila studies used a very small number of nuclear and mitochondrial genotypes. Therefore, we don’t know if the Mother’s Curse is generalisable across species like Drosophila that have high levels of genetic variation!
The solution? We investigated the presence of the Mother’s Curse using a mito-nuclear genetic panel produced through the pairwise-combination of nine populations of Drosophila melanogaster drawn from around the world. Consequently, this panel contained 81 unique mito-nuclear combinations – very large! We then screened each genotype and sex combination for wing centroid size, a proxy of Drosophila body size. We chose this trait because it exerts high metabolic demands and there is evidence of divergent selection on the trait in the two sexes.
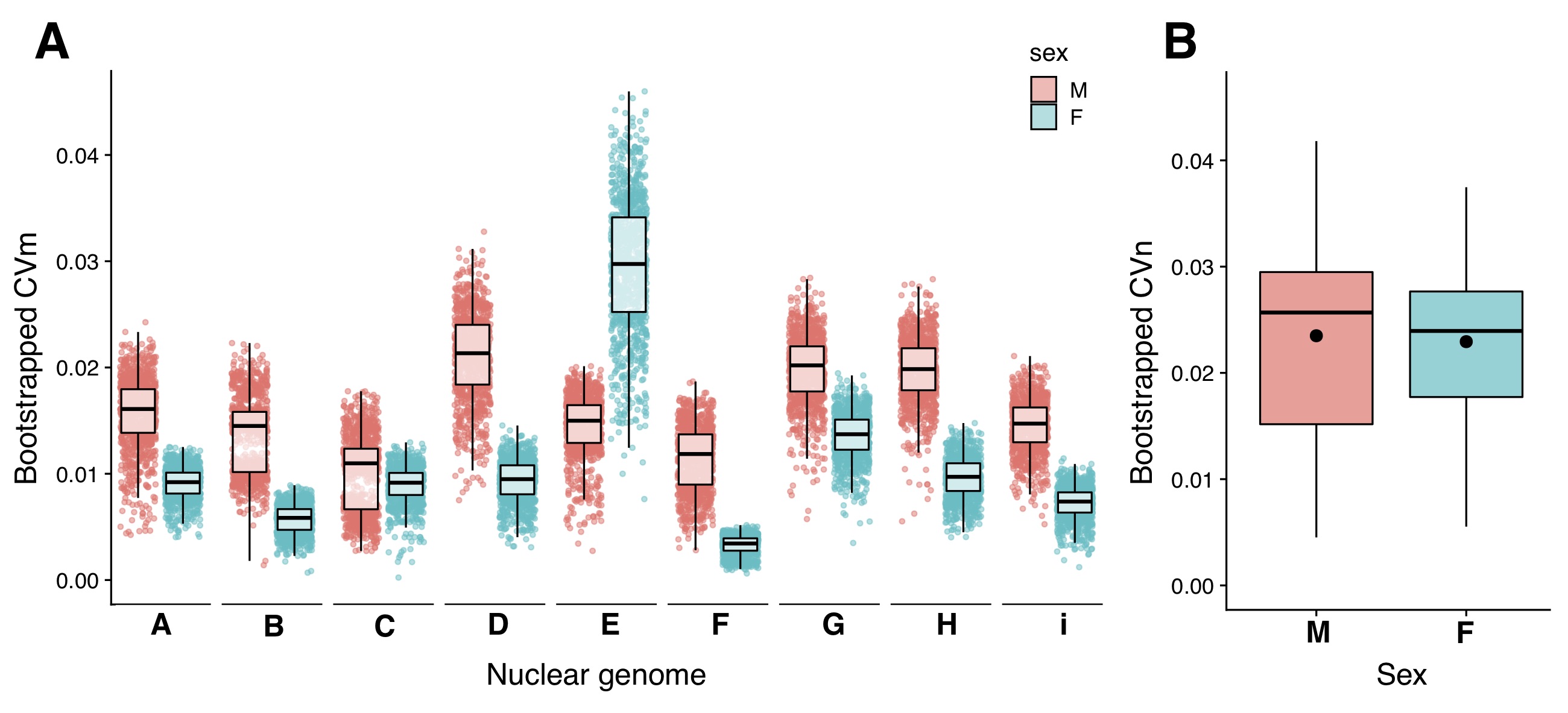
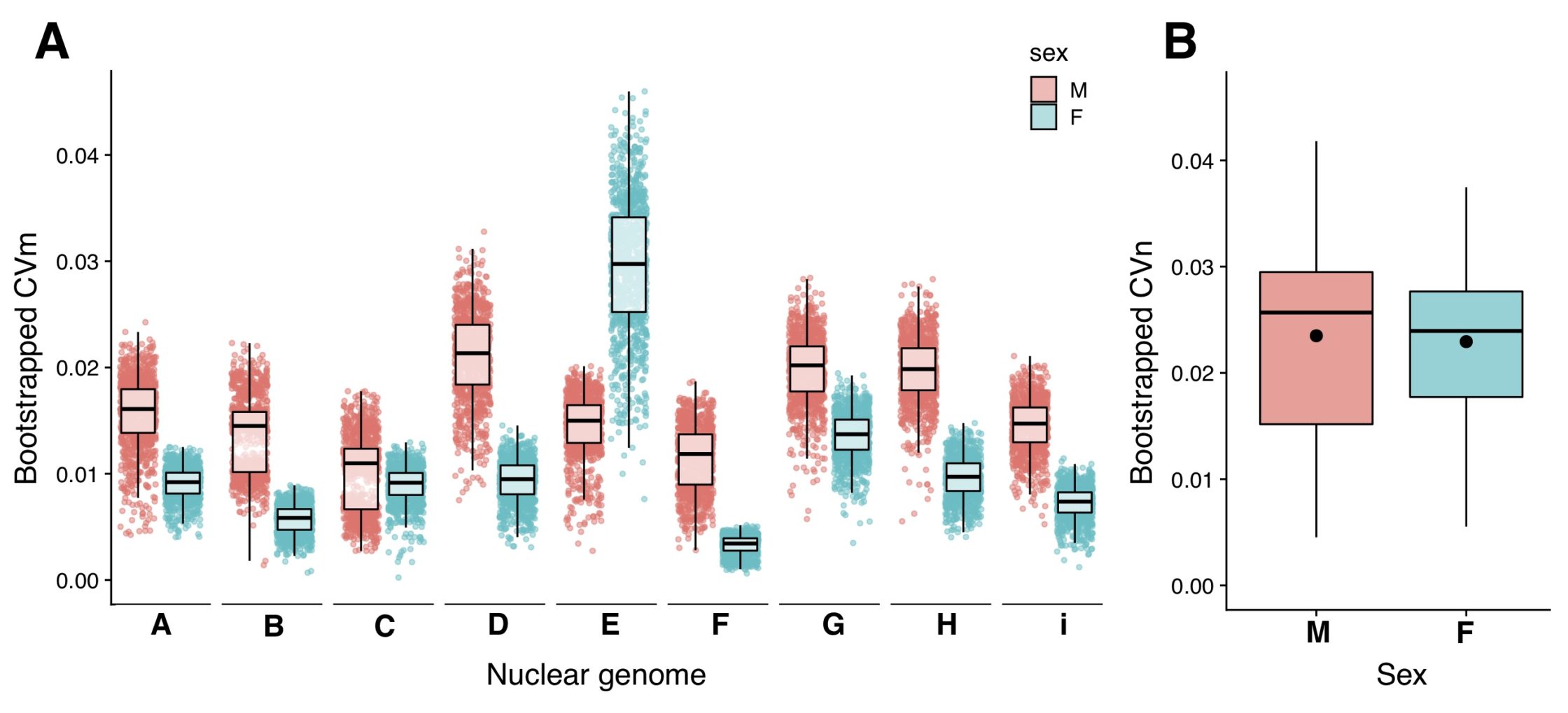
Our study’s results support the Mother’s Curse hypothesis in two ways (but please see the publication for more details). First, males showed higher levels of mitochondrial genetic variance (measured as a coefficient of variation, or CVm) than females in eight of the nine nuclear backgrounds used in this study (Fig. 2 – A). Secondly, the variance accounted for by mitochondrial DNA genotypes in our linear models is greater in males than females (Fig. 2 – B). Together, these results demonstrate that the Mother’s Curse is more pervasive than previously appreciated. The panel will now allow us to test further predictions of the hypothesis, such as how the degree to which the signature of the Mother’s Curse depends on the trait’s metabolic burden.
Lorcan Carnegie is a PhD researcher who worked with Dr Florencia Camus at University College London. The original article is freely available to read and download from Evolution Letters.