Pinning down how organisms interact is more complicated than you think. The introductory biology version of interactions between species is that they fall into neat categories depending on who is helped or harmed. For example, ants protect some species of plants from herbivores. The plants return the favor by releasing sugary substances to feed the ants. Since both ants and plants benefit, this kind of interaction would be called a mutualism. In contrast, if one side benefits at the expense of the other, like when a virus exploits its human host, the interaction would be called antagonism. However, most interactions (and even the ant-plant and virus-human examples) are rarely this tidy because interactions can shift from mutualistic to antagonistic and vice versa in different environments. Our study shows that to be the case for both sides of a symbiosis between Dictyostelium discoideum amoebae and the Paraburkholderia bacteria that live inside them (these are called “symbionts”). We also show that these shifting interactions do not fundamentally change the story of how interactions between species evolve.

D. discoideum are amoebae that live in the soil and eat bacteria. When D. discoideum runs out of food bacteria, it forms a fruiting body — a structure for dispersing spores that will become new amoebae to new environments. Amoebae and their fruiting bodies are hosts to three species of symbiotic Paraburkholderia bacteria that catch a ride with hosts. These Paraburkholderia symbionts are inedible to the hosts but are more than just stowaways; they provide hosts with an important benefit. Hosts that have Paraburkholderia symbionts can carry food bacteria when they disperse to new environments. Being able to carry food bacteria allows hosts to pack their lunch when dispersing. This is a major benefit for hosts when food bacteria are rare but comes with a cost when food bacteria are already in an environment. This means that this symbiosis has elements of mutualism and antagonism for hosts depending on the amount of food bacteria.
What about the Paraburkholderia symbionts?
Most of the research on this symbiosis has focused on the host side of the story. The side of Paraburkholderia symbionts has been more of a mystery. One important question is whether Paraburkholderia symbionts are affected by food bacteria in the environment. That is the first question we set out to test in this study.
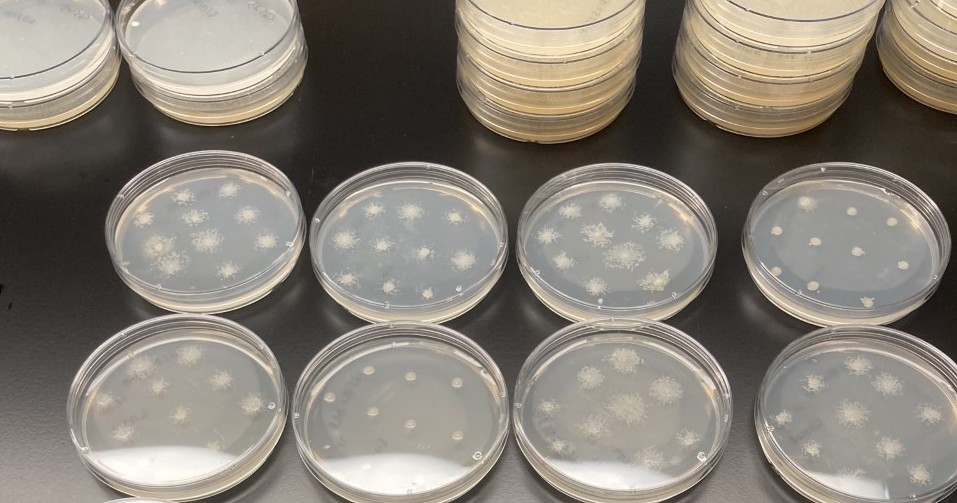
To test how food bacteria affect the symbionts, we measured the amount of Paraburkholderia after dispersing host spores that contained food bacteria and Paraburkholderia to new environments. These new environments (agar plates) could either contain or lack food bacteria. We found that, like their hosts, Paraburkholderia symbionts are affected by the presence of food bacteria. In this case, symbionts benefit when there are fewer food bacteria taking up resources.
We wondered if the amount of Paraburkholderia in these different environments affected the hosts. By measuring the number of symbionts and the number of spores produced by hosts, we found that Paraburkholderia tended to harm hosts, but less so when they were on plates without food. This indicates that while this interaction involves some antagonism, there may be some environments that involve more mutualism.
Does an environment that changes over time modify how symbioses evolve?
We expect that D. discoideum will encounter environments with and without food bacteria over time as it eats, forms fruiting bodies, and disperses in the soil. This means that the interaction between D. discoideum and Paraburkholderia will change over time. Interactions that change in this way may modify our thinking about the evolution of mutualisms more generally. Usually, we expect mutualistic interactions to evolve when the benefits of interacting outweigh the costs so that both sides benefit. However, the calculation of costs and benefits changes when conditions change over time because rare, harsh conditions that drastically reduce growth can have outsized impacts on long-term growth. To avoid these drastic reductions in growth, some organisms “hedge their bets” with phenotypes that avoid the impact of the harshest conditions but that are less well adapted for good conditions. The same logic may apply to symbioses where hosts will hedge their bets by partnering with symbionts that reduce growth when conditions are good and provide a benefit when conditions are bad.
We tested whether having a symbiont was beneficial for D. discoideum hosts because of either the traditional explanation of more benefits than costs or the explanation of bet-hedging. We used our empirical data to simulate how hosts are affected in environments where the amount of food bacteria changed across time. When food bacteria are frequently common, conditions are good for hosts. When food bacteria are frequently rare, conditions are harsh. Our simulations showed that symbiosis was often favored, especially in harsh conditions. However, when having symbionts was advantageous, it was often because the benefits outweighed costs. Bet-hedging only appeared in a few cases. This shows that interactions can be beneficial in harsh and variable environments, but this does not modify how interactions should evolve.
Together, our results show that the abundance of food bacteria matters for both D. discoideum hosts and Paraburkholderia symbionts. However, our simulation results show that bet-hedging is probably not required for D. discoideum hosts to benefit in harsh conditions from their association with Paraburkholderia. Instead, the conventional explanation of costs outweighing benefits fits best for this symbiosis.



1 Comment
Fascinating post. I would be interested to know how Paraburkholderia enables the slime mold to carry their food bacteria with them when they disperse.